Eine führende Technologie-Marke
neuroConn ist unsere führende Technologiemarke aus Deutschland. Seit fast zwanzig Jahren steht unsere Marke für praxisorientierte Medizintechnik, die in der neurowissenschaftlichen und klinischen Forschung sowie in der Diagnostik und Therapie neuropsychologischer Störungen eingesetzt wird.
In enger Zusammenarbeit mit führenden Forschungseinrichtungen und Kliniken weltweit wird die neuroConn-Technologie nach den neuesten Standards der medizinischen Forschung entwickelt und bietet den Anwendern standardisierte und klinisch evaluierte Protokolle.

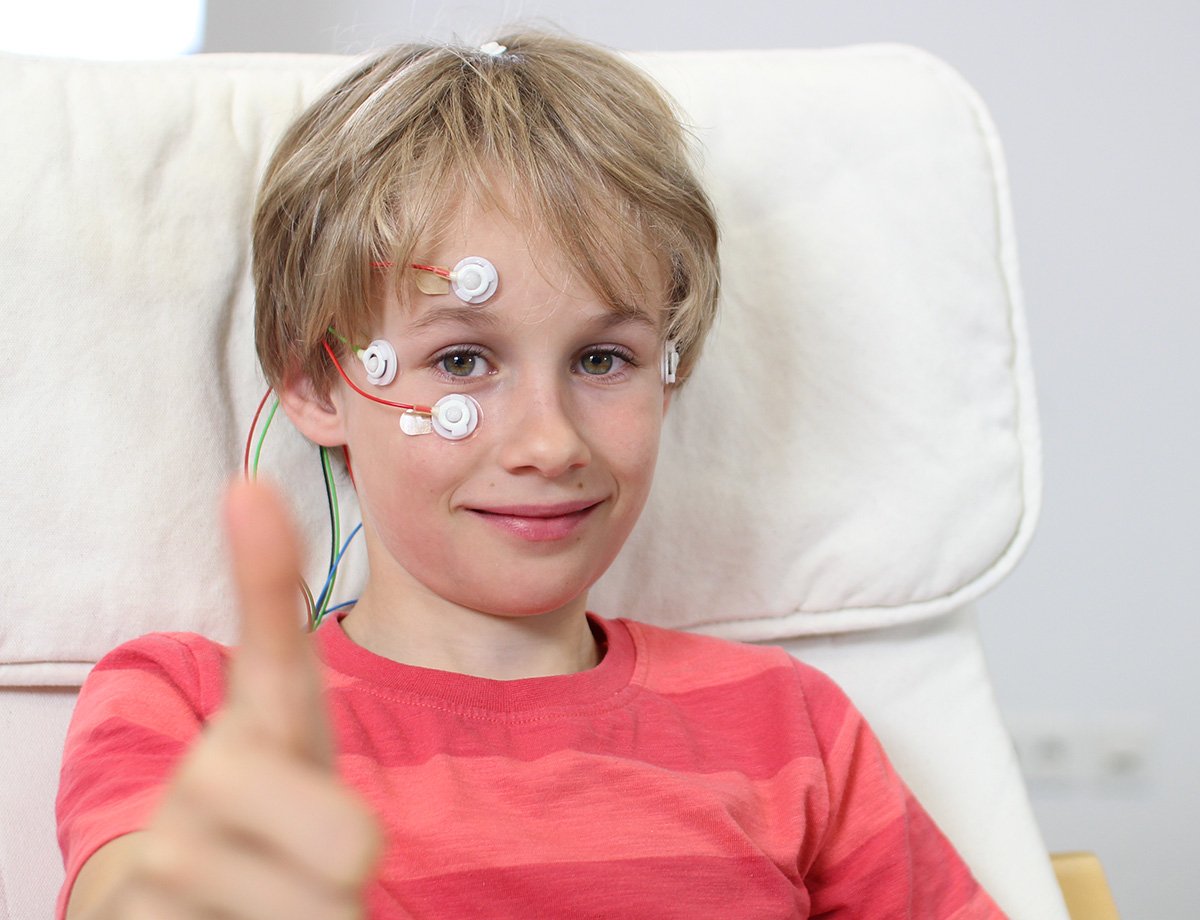
Bewährt in der Forschung
neuroConn DC-STIMULATOR-Geräte und die NEURO PRAX® full-band-EEG-Systeme werden in klinischen Studien und neurowissenschaftlichen Untersuchungen in führenden Forschungsinstituten weltweit eingesetzt.
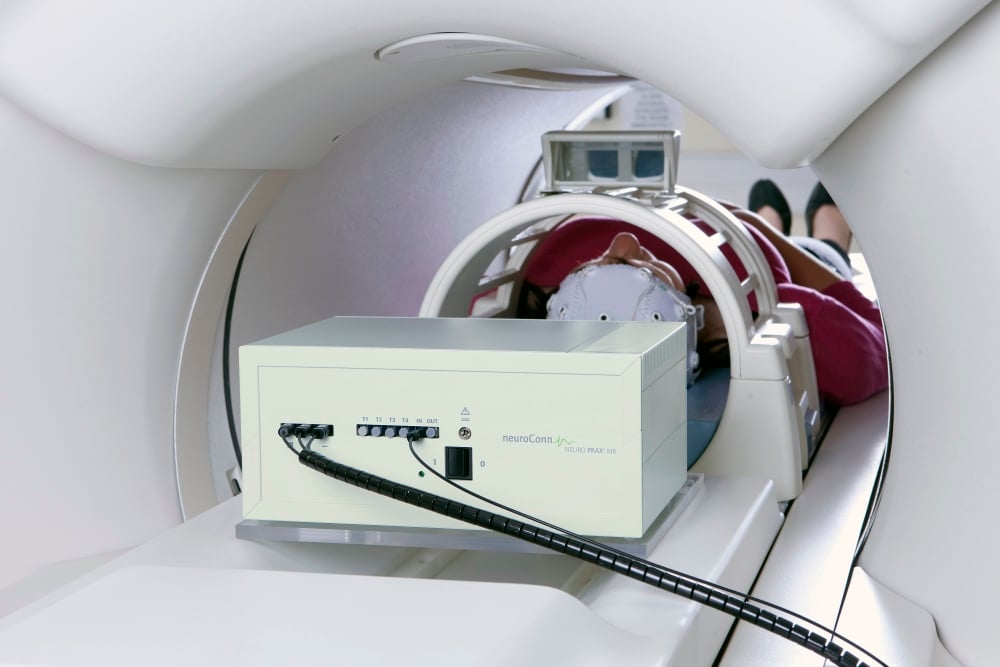
Neuromodulation für die Therapie
Unsere Technologie sind in vielen Kliniken und Praxen weltweit im Einsatz. Die Geräte helfen Patient:innen bei mentalen Störungen, bei chronischen Schmerzen und in der Rehabilitation.
THERA PRAX® bietet Ihnen evidenzbasiertes SCP-Neurofeedback nach wissenschaftlichen Erkenntnissen. Der DC-STIMULATOR verbessert die Aktivität der betroffenen Gehirnareale durch schwachen Gleichstrom. Das neuroLAB bietet ein Labor für neuropsychologische Tests und quantitative EEG-Analyse aus einer Hand.

neuroConn Produkte und Lösungen
THERA PRAX® Serie
Neurofeedback-Systeme mit präziser Online-Artefaktkontrolle. Ideal für SCP-Neurofeedback und Neurofeedback der Frequenzbänder, mit optionalen Biofeedback-Erweiterungen.
DC-STIMULATOR
Nicht-invasive elektrische Gehirnstimulation mit tDCS/tACS/tRNS für Therapie und Forschung. Je nach Gerätetyp einkanalig oder mehrkanalig, für Anwendung während Bildgebungsverfahren.
NEURO PRAX® Serie
Full-band DC-EEG-Systeme mit 8/32/64/128 Kanälen. Ideal für rauschfreies EEG im fMRT und während transkranieller Gehirnstimulation (TMS/tDCS/tACS/tRNS). Für die Forschung.
neuroLAB
QEEG-Lab für topografische Analysen und psychometrische Testung. Für die Auswahl der Neurofeedback- und Neurostimulationsprotokolle und für personalisierte Medizin.
News aus dem Bereich der neuroConn Technology
Lesen Sie hier aktuelle Infos zur Neuromodulation, Gerätetechnik, Studienlage, Fortbildungen, Events und vieles mehr.
Aktuelle Förderprojekte
Elektrisch stimulierte Atmung - ElekSA
(2021 VF 0036)
Vom Freistaat Thüringen gefördertes Vorhaben, durch Mittel der Europäischen Union im Rahmen des Europäischen Fonds für regionale Entwicklung (EFRE) und von REACT-EU kofinanziert.



